
MULTIPLEX ASSAY DEVELOPMENT WITH XMAP® TECHNOLOGY WHITE PAPER
Abstract
Multiplex Assay Development with xMAP® Technology at RBM
![]() Our internally developed Multi-Analyte Profiles (MAPs) have the ability to accurately quantify a few or hundreds of biomarkers using only a small volume of valuable clinical sample.
Our internally developed Multi-Analyte Profiles (MAPs) have the ability to accurately quantify a few or hundreds of biomarkers using only a small volume of valuable clinical sample.
Utilizing decades of experience developing and manufacturing immunoassays for a vast array of biomarker targets, our expertise in assay development methodology sets us apart from other protein biomarker testing laboratories. Here we highlight the procedures implemented by RBM that take Luminex xMAP® technology to a new level of reproducibility and ruggedness.
Our processes and validation parameters result in the highest quality assays and allow us to rigorously maintain that quality year after year, a vital component in our efforts to support the clinical trial needs of our customers.
Multiplex Assay Development Workflow
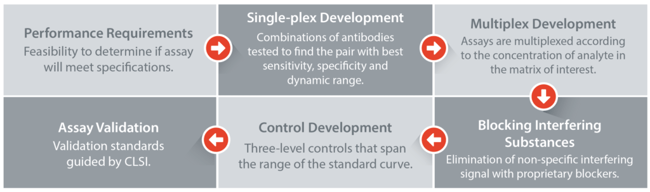
xMAP Technology Advantages and Benefits

